Incredible! Why Is Nitrogen More Basic Than Oxygen
First and foremost because theres a lot of nitrogen in the atmosphere. That means that electrons are not held so tightly in N as.

Pin On Mcat Practice Questions
Secondly nitrogen gas is an incredibly stable molecule possibly THE most stable molecule.
Why is nitrogen more basic than oxygen. All else being equal in similar bonding situations nitrogen is a stronger base than oxygen because its less electronegative. Oxygen gas on the other.

Factors That Determine Acid Strength Mcc Organic Chemistry

5 Key Basicity Trends Of Amines Master Organic Chemistry
How To Identify The Most Basic Atom

5 Key Basicity Trends Of Amines Master Organic Chemistry
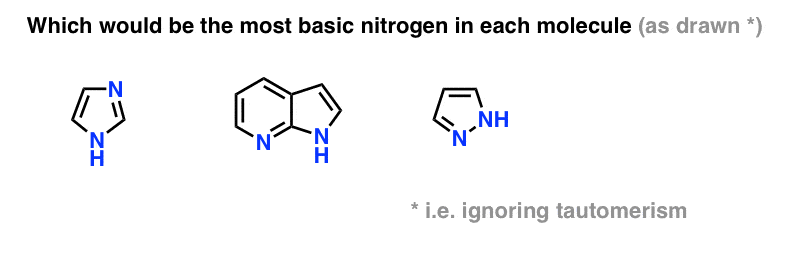
5 Key Basicity Trends Of Amines Master Organic Chemistry
Is Nitrogen More Electronegative Than Oxygen Quora

Factors That Determine Acid Strength Mcc Organic Chemistry
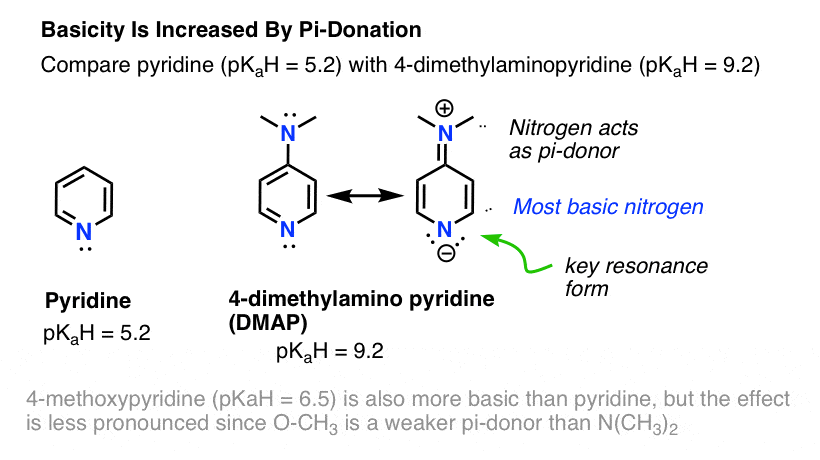
5 Key Basicity Trends Of Amines Master Organic Chemistry

5 Key Basicity Trends Of Amines Master Organic Chemistry Organic Chemistry Chemistry Pi Bond
Post a Comment for "Incredible! Why Is Nitrogen More Basic Than Oxygen"