WOW..Why Is Hcl Stronger Than Acetic Acid
B Arrange the four. Ii The pH of solution A is 6 B is 9C is 12 and D is 7.
Why Choloroacetic Acid Is More Acidic Than Acetic Acid Quora
HCL is stronger than acetic acid because it undergoes almost complete ionisation when dissolved in waterie.

Why is hcl stronger than acetic acid. How will you verify it. 18 i Explain why HCl is a stronger acid than acetic acid. Formation of H and Cl- ion whereas when acetic acid is dissolved in water only 5 of it.
A Identify the most acidic and most basic solutions. Acid strength depends upon ease of ionization of the acidic hydrogen forming hydronium ions by proton transfer. HCl is one of the strong acids and as such ionizes 100 whereas Acetic Acid.

I Explain Why Is Hydrochloric Acid A Strong Acid And Acetic Acid A Weak Acid How Can It Be Verfied Ii Explain Why Equeous Solution Of An Acid Conducts Electricity

Explain A Acetic Acid Is A Stronger Acid Than Ethyl Alcohol Youtube

Solved 18 I Explain Why Hcl Is A Stronger Acid Than Acetic Acid How Will You Verify It Ii The Ph Of Solution A Is 6 B Is 9 C Is 12 And

I Explain Why Is Hydrochloric Acid A Strong Acid And Acetic Acid A Weak Acid How Can It Be Verfied Ii Explain Why Equeous Solution Of An Acid Conducts Electricity
Why Is Acetic Acid A Stronger Acid Than Ethyl Chegg Com
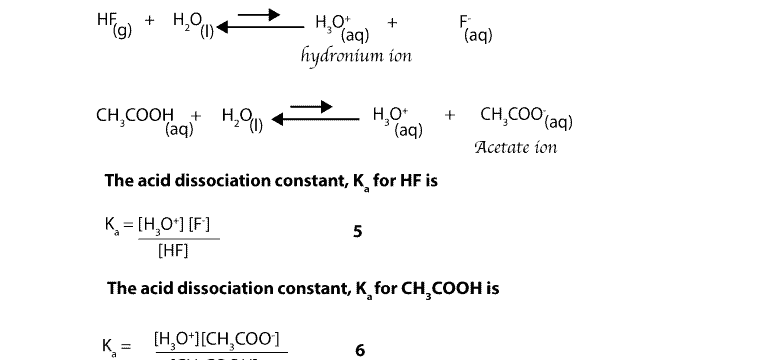
Why Re Hydrochloric Acid Nitric Acid And Sulfuric Acid Strong Acids While Hydrofluoric Acid And Acetic
How Does Acetic Acid React To Hydrochloric Acid Quora
Why Choloroacetic Acid Is More Acidic Than Acetic Acid Quora
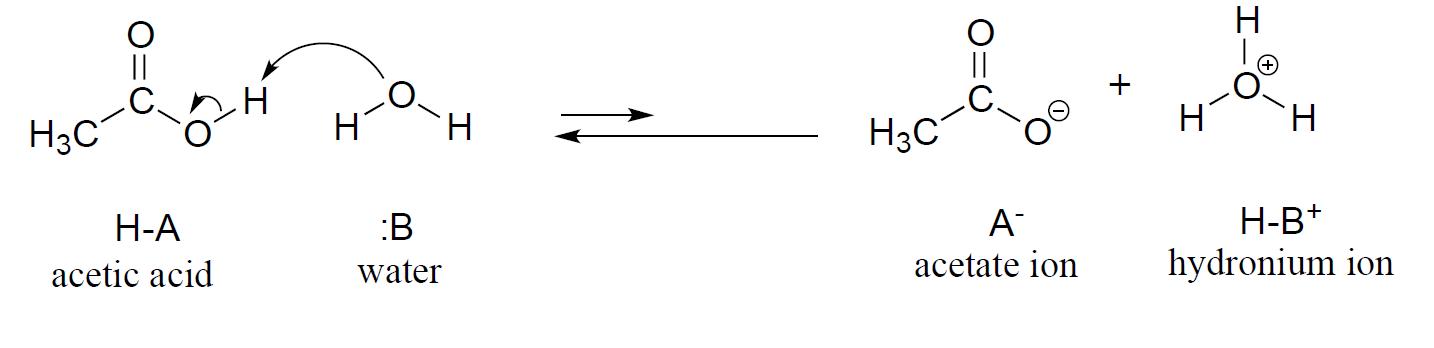
2 8 Acid And Base Strength Chemistry Libretexts

Post a Comment for "WOW..Why Is Hcl Stronger Than Acetic Acid"