OH MY GOD! Why Do Noble Gases Have High Ionization Energies
Why do noble gases have the highest first ionisation energy. An obvious feature of this figure is that the elements with the highest ionization energies are the noble gases.
The Noble Gases Group 18 Introduction To Chemistry
Ionization energy can be thought of as the energy required to remove an electron from the valence shell of an atom.

Why do noble gases have high ionization energies. Since noble gases have completely filled valence shells. Since noble gases have completely filled valence shells that are highly stable a lot of energy would be required to remove even a single electron from the valence shell of a noble gas. Why do noble gasses have high ionization energy.
Since the ionization energy measures the energy which must be supplied to remove an electron.
Noble Gases And Other Atoms Jealousy Of Them The Bumbling Biochemist
8 2 Periodic Trends Physical Science
Do Noble Gases Have High Or Low Ionization Energies Socratic
High School Chemistry Electron Affinity Wikibooks Open Books For An Open World
Why Is The Ionisation Energy Of Noble Gases Exceptionally Higher Quora
Comparision Of The Ionisation Energy Change Of The Alkali Metals And Noble Gases Chemistry Stack Exchange
Chemistry Homework Read Pp Problems P 141 1 2 3 5 10 12 13 Ppt Download
Chemistry The Central Science Chapter 7 Section 3
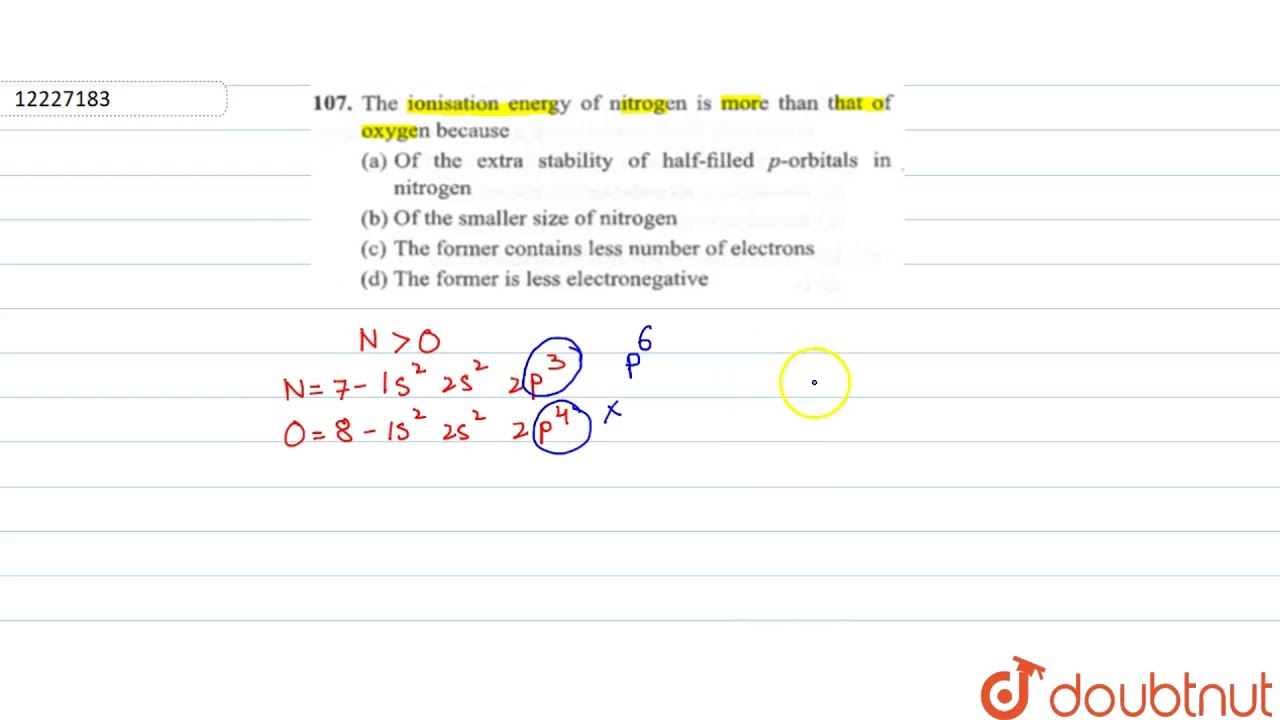
Post a Comment for "OH MY GOD! Why Do Noble Gases Have High Ionization Energies"