GREAT..Why Nitrogen Has Higher Ionization Enthalpy Than Oxygen
Ionisation potential increases across a period from left to right. The second ionization energies in kJmol 1 of some elements of period of 4 are.
The Ionisation Energy Of Nitrogen Is More Than That Of Oxygen Because Youtube
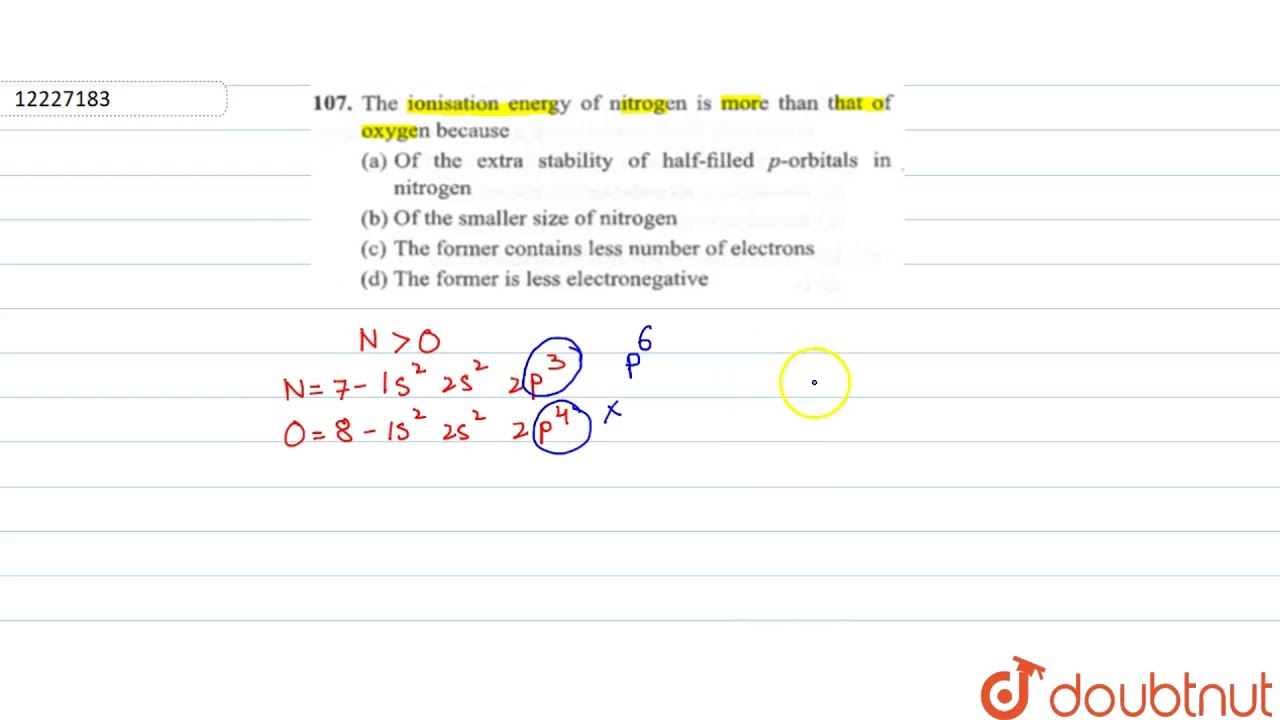
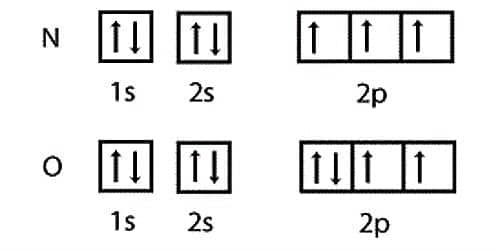
As written above the half-filled configuration is just below the completely filled configuration in terms of stability so the stability of nitrogen atom is more as compared to oxygen and.
Why nitrogen has higher ionization enthalpy than oxygen. The valency shell is exactly half filled which develops degeneracy which leads to little stabilityaccording to Hunds rule than Oxygen atom which has an electronic configuration of 1s2 2s2 2p4 which do not have degeneracy like Nitrogen atom.
Why The First Ionization Potential Of Nitrogen Is Higher Than That Of Oxygen Qs Study

Atomic Nitrogen Has A Higher Ionization Energy Than Atomic Oxygen This Is Best Explained By Youtube
Why Does Nitrogen Have A Higher Ionization Energy Than Carbon Quora
Why Is The First Ionization Energy Of Nitrogen More Than Oxygen Quora
Why Is The First Ionization Energy Of Nitrogen More Than Oxygen Quora

Nitrogen Has A Electron Value Of 3 0 Nitrogen Electrons Periodic Table

Is The First Ionization Energy In Oxygen Slightly More Than Nitrogen Chemistry Stack Exchange
Why Nitrogen Needs More Ionization Energy Than Oxygen Quora
Post a Comment for "GREAT..Why Nitrogen Has Higher Ionization Enthalpy Than Oxygen"