Perfect! Why Does Water Have A Higher Boiling Point Than Expected
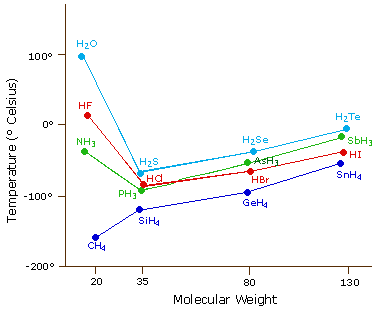
Water has a high boiling point because its molecules are bound together by hydrogen bonding which is a very strong intermolecular force. Water molecules in liquid state have the ability to form hydrogen bonds with each other.
The greater the forces of attraction the higher the boiling point or the greater the polarity the higher the boiling point.

Why does water have a higher boiling point than expected. The water molecule has an EXCEPTIONALLY high boiling point compared to molecules of comparable or even much larger size due to the existence of potent and characteristic intermolecular forces. It takes more kinetic energy or a higher temperature to break the hydrogen bonding between water molecules. These hydrogen bonds are some of the strongest of all intermolecular forces so a large amount of energy is needed to break these interactions.
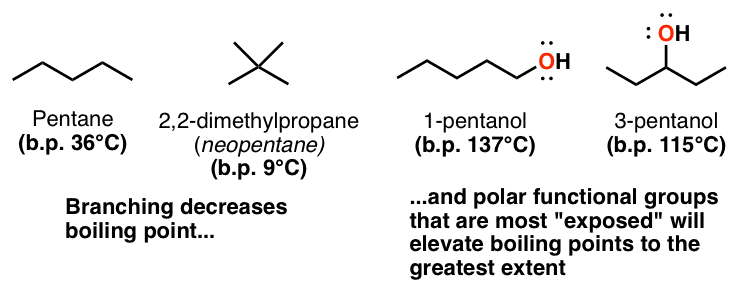
3 Trends That Affect Boiling Points Master Organic Chemistry
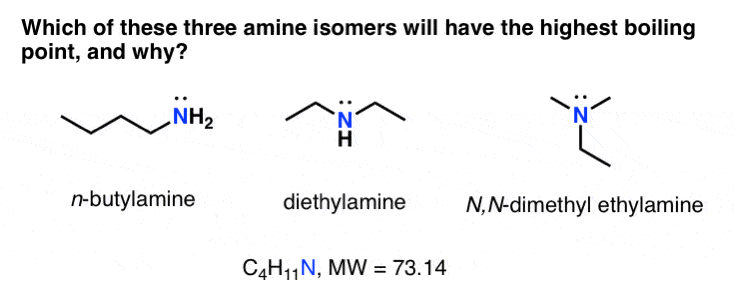
3 Trends That Affect Boiling Points Master Organic Chemistry
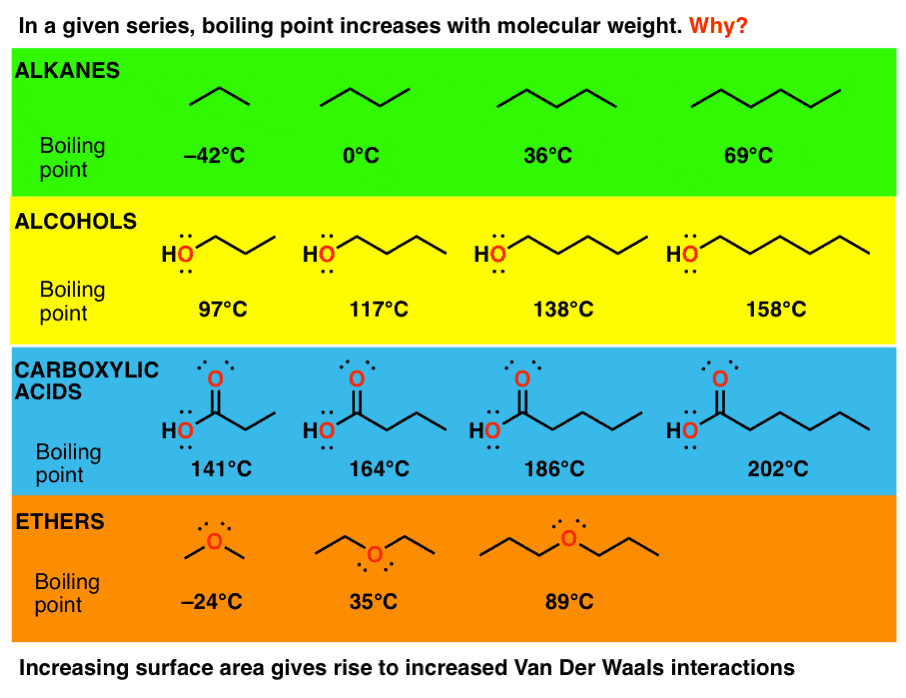
3 Trends That Affect Boiling Points Master Organic Chemistry
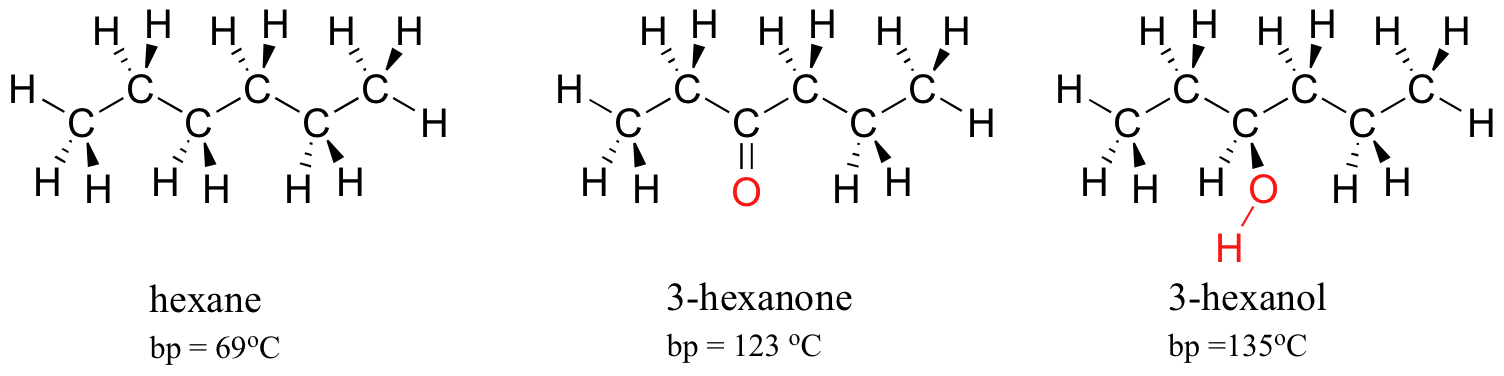
2 11 Intermolecular Forces Relative Boiling Points Bp Chemistry Libretexts

Why Does Water Has A Higher Boiling Point Than Hf Hydrogen Bonding In Water And Hydrogen Fluoride Youtube
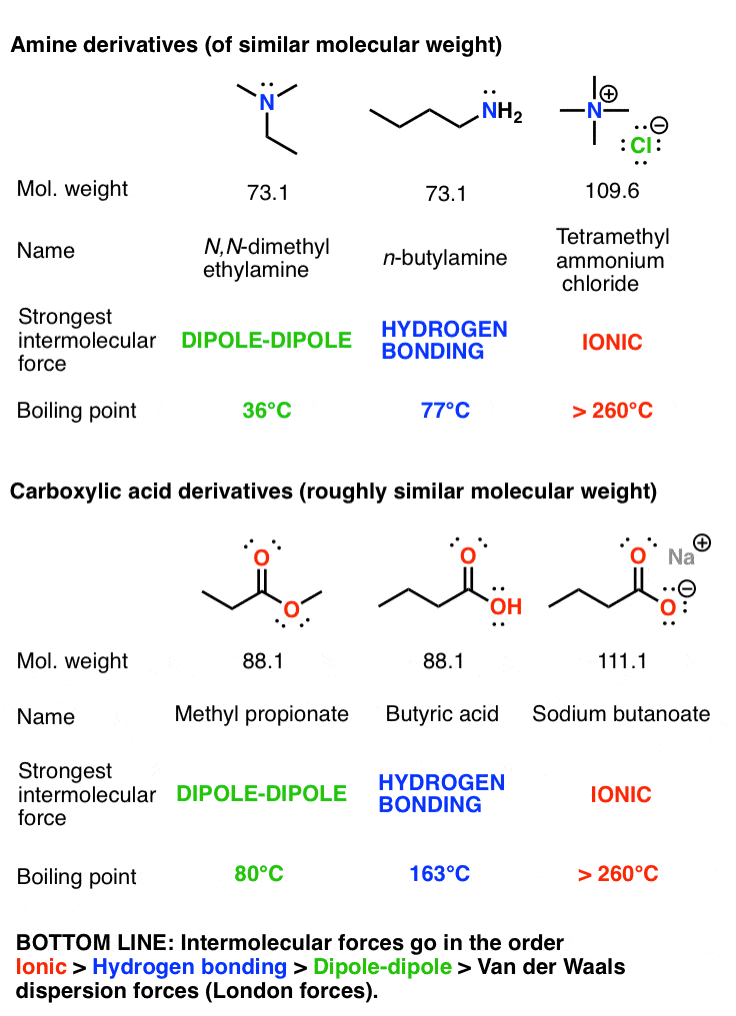
3 Trends That Affect Boiling Points Master Organic Chemistry

3 Trends That Affect Boiling Points Master Organic Chemistry
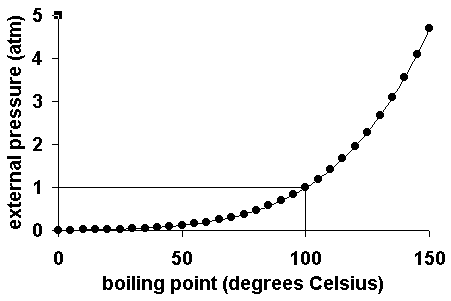
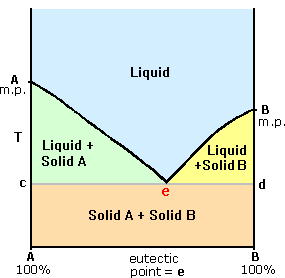
Post a Comment for "Perfect! Why Does Water Have A Higher Boiling Point Than Expected"