Amazing ! Why Is Hcl A Stronger Acid Than Acetic Acid
It almost completely dissociates in water and its value of Ka is very large around 13 106. The general trend for hydrohalic acids is that acidity.

I Explain Why Is Hydrochloric Acid A Strong Acid And Acetic Acid A Weak Acid How Can It Be Verfied Ii Explain Why Equeous Solution Of An Acid Conducts Electricity
In water the strongest acid that can exist is H3O or Increased electronegativity doesnt translate to stronger bonds.

Why is hcl a stronger acid than acetic acid. The strength of an acid is determined by how much of theacid ionises. Hydrochloric acid is a strong acid which means it gets completely ionized into H Cl- which in turn means that all the H present in HCl will be present in solution now. Acid strength depends upon ease of ionization of the acidic hydrogen forming hydronium ions by proton transfer.
HCl gives more H but acetic acid gives less H ions as. A Identify the most acidic and most basic solutions. How will you verify it.
The long answer - you need to consider the pKa values for the two acids and remember that the smaller the pKa value the stronger the acid. But in case of acetic acid it is not so. Of your list HCl is the strongest acid followed by acetic acid ammonia and sodium hydroxide.
I want to know why HCl is a much stronger acid than H2OThey have similar bond energies H-Cl 427 kJmol O-H 467 kJmol so there should be easy to ionize both. Much of the time chemists measure the strength of an acid. The pKa values are shown below.
The difference lies in there ionization. B Arrange the four. HCl produces high concentration of hydronium ion compared to that of concentrated acetic acid.
HCl acid ionises completely to H and Cl - ions that is about 100 out of 100 molecules donate their hydrogen ion. Ii The pH of solution A is 6 B is 9C is 12 and D is 7. But what makes the HCl is stronger than H2SO4 is the difference in Basicity of both acids.
Due to - i effect of chlorine atom chloroacetic acid is strongerthan acetic acid as conjugate base chloroacetate is much stablethan acetate anion. According to ionising with water HCl completely ionised whereas acetic acid partially ionised. Strength of an acid is the measure of concentration of hydronium ions it produces in its aqueous solution.
Acetic acid on the other hand dissociates partially. Hence it is a strong acid. Hydrochloric acid is a stronger acid than Acetic acid for thesereasons.
Formation of H and Cl- ion whereas when acetic acid is dissolved in water only 5 of it. HCl is a strong acid because it has more number of hydrogen ions whereas acetic acid contains less number of hydrogen ions so its a weak acid it can be varied by changing number of hydrogen ions in. HCl is a strong acid because it dissociates almost completely.
Chloroacetic acid pKa 287. In contrast the HCl is monoprotic acid whereas the H2SO4 is Diprotic acid. As it is a weak acid it dont get ionized completely.
So chloroacetic acid has the smallest pKa and is therefore the stronger acid. Besides O is more electronegative than. Hencethe number of H.
Hydrochloric is an acid which. HCL is stronger than acetic acid because it undergoes almost complete ionisation when dissolved in waterie. HCl is one of the strong acids and as.
In contrast the HCl is monoprotic acid whereas the H2SO4 is Diprotic acid. Hydrochloric acid an ionic compound formed with hydrogen and chlorine is a very strong acid with a pH level depending on the concentration wavering between -1 and 1. By contrast a weak acid like acetic acid CH3COOH does not dissociate well in water many H ions remain bound-up.
Formic acid pKa 375. Hydrochloric acid is stronger than hydrofluoric acid. The stronger an acid the more it dissociates to form hydrogen ions in a solvent.
When the same concentration of HCI and acetic acid are taken one molar then these produce different amounts of H ion as HCI dissociates completely. 18 i Explain why HCl is a stronger acid than acetic acid. For the presence of 3 chlorine.

Strong Weak Acids Bases Mr Carson S Science Page
How Does Acetic Acid React To Hydrochloric Acid Quora
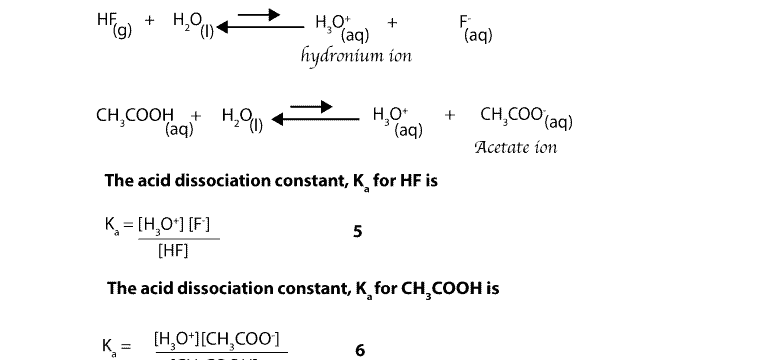
Why Re Hydrochloric Acid Nitric Acid And Sulfuric Acid Strong Acids While Hydrofluoric Acid And Acetic

Why Re Hydrochloric Acid Nitric Acid And Sulfuric Acid Strong Acids While Hydrofluoric Acid And Acetic Acid Weak Acids
Why Choloroacetic Acid Is More Acidic Than Acetic Acid Quora

I Explain Why Is Hydrochloric Acid A Strong Acid And Acetic Acid A Weak Acid How Can It Be Verfied Ii Explain Why Equeous Solution Of An Acid Conducts Electricity
Why Choloroacetic Acid Is More Acidic Than Acetic Acid Quora
Why Is Hcl A Stronger Acid Than Acetic Acid Quora

Explain A Acetic Acid Is A Stronger Acid Than Ethyl Alcohol Youtube
Post a Comment for "Amazing ! Why Is Hcl A Stronger Acid Than Acetic Acid"