Helpful! Why Silicon Dioxide Reacts With Calcium Oxide
Silicon dioxide reacts with calcium oxide to form calcium silicate called slag which is liquid in the furnace. The calcium oxide then reacts with silica sand impurities in the haematite to produce slag which is calcium silicate.
![]()
Solubility Of Calcium Hydroxide And Silicon Dioxide In Water At Download Scientific Diagram
- Chemistry Stack Exchange.

Why silicon dioxide reacts with calcium oxide. Slag flows to the bottom of the furnace Acid-base Behavior of the Oxides Chemistry. Acid base - Why is the reaction between calcium oxide and silicon dioxide neutralization. Why does silicon dioxide react with calcium oxide.
I get that calcium oxide is a basic oxide and silicon dioxide is acidic. However by definition neutralization is when an acid and a base react to form water and a salt.

How To Balance Cao Sio2 Casio3 Calcium Oxide Silicon Dioxide Youtube
Learn Quick Lime Preparation Properties And Uses In 4 Minutes

How To Balance Cao Sio2 Casio3 Calcium Oxide Silicon Dioxide Youtube

How To Balance Cao So2 Caso3 Calcium Oxide Sulfur Dioxide Youtube
![]()
Topic 3 Metals And Metal Extraction Ppt Download

Topic 3 Metals And Metal Extraction Ppt Download
![]()
Solubility Of Calcium Hydroxide And Silicon Dioxide In Water At Download Scientific Diagram
5 1 Writing And Balancing Chemical Equations Problems Chemistry Libretexts
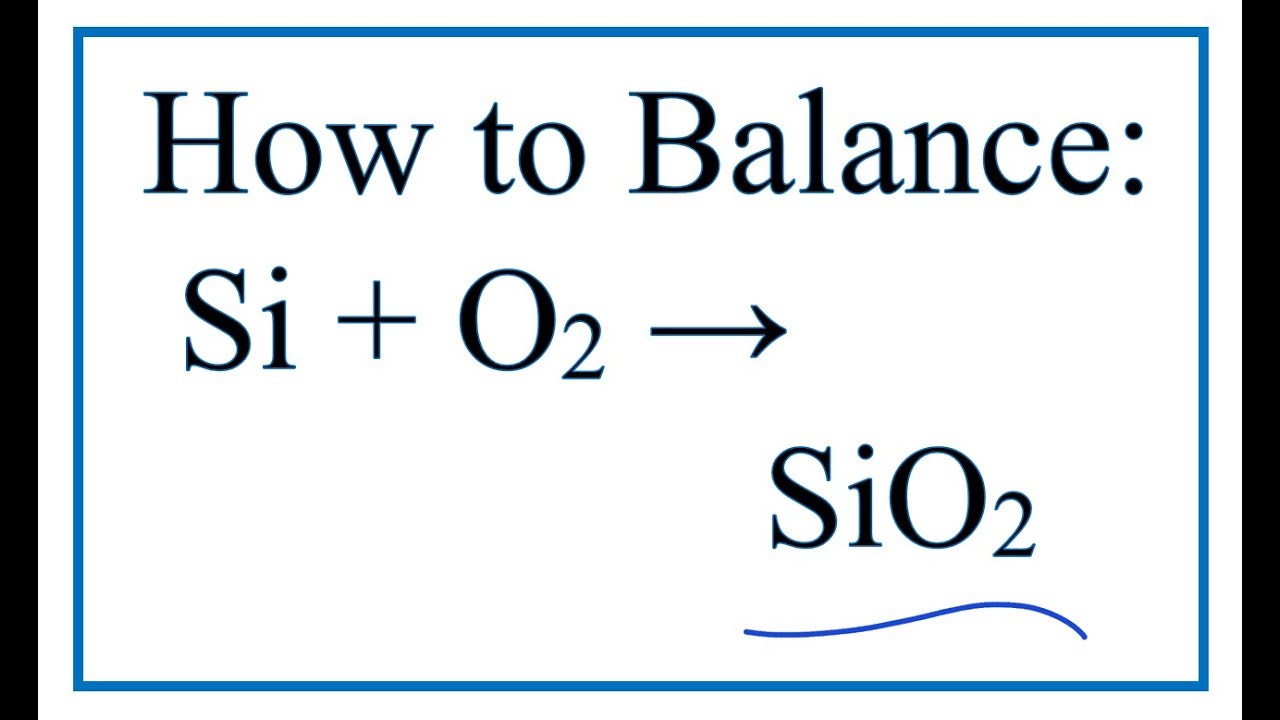
How To Balance Si O2 Sio2 Silicon Oxygen Gas Youtube
Post a Comment for "Helpful! Why Silicon Dioxide Reacts With Calcium Oxide"